|
What is a plasma? 

The planets, air, water, stones and living creatures, all natural bodies are made up of atoms (or assemblies of atoms called molecules). In its turn, an atome
 is made up of :
is made up of :
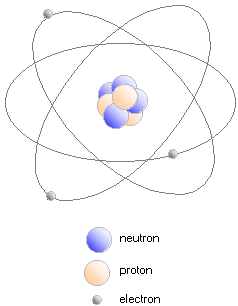 |
- a central positively charged kernel, which is an assembly of protons and neutrons.
- a peripheral cloud made up of a series of negatively charged electrons, which hurtle round the kernel at prodigious speeds.
|
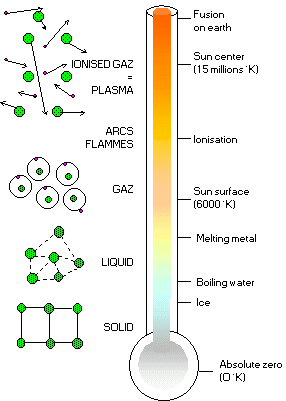
| In the solid state, the atoms are firmly imprisoned inside a rigid network (like ice for example). When we raise the temperature, they go into a liquid state (the ice melts), where the atoms may slide around in relation to the others, thus enabling a liquid to adapt to the shape of a container. If we heat it up even more, we arrive at the gas state: atoms then move around freely independently of each other (water turns into steam). Finally, when we get to very high temperatures (typically several million degrees!), the ingredients of the atom separate, kernels and electrons move around independently and form a globally neutral mixture: this is a plasma (nothing to do with blood plasma!). |
 |
|

|
This fourth state of matter, found in the stars and the interstellar environment, makes up most of our universe (around 99 %). On Earth, it does not exist in a natural form (you might wonder how we manage to live in the remaining 1%!), apart from in lightning and the Aurora Borealis, but it is produced artificially by applying magnetic fields powerful enough to separate the kernel from its electrons in gases. In our everyday life, plasmas have many applications (micro-electronics, television flat screens and so on), of which the commonest is the neon tube in our bathrooms.
|
Depending on the temperature, the atoms may be partially or wholly ionised (i.e. the kernel is partially or wholly "peeled" of its electrons). A plasma may thus be considered as a mixture of positively charged ions and negatively charged electrons, possibly co-existing with neutral atoms and molecules. For example, in our luminescent tube, the ions and electrons in a small proportion in relation to atoms and molecules. On the other hand, in plasmas produced for fusion experiments, the gas is strongly ionised, and the atoms and molecules are in low proportion, even completely absent in the heart of the pulse. In both cases, the description of plasmas comes from the physics of gases and fluid mechanics, and it uses usual macroscopic features such as density, temperature, pressure and rate of flow.
|
|